As the COVID-19 pandemic continues to spread in many parts of the world, there is an urgent need for a preventative vaccine and improved therapeutics. A new study by researchers at NYU Langone Medical Center and Weill Cornell Medical College and published on the preprint server bioRxiv* in September 2020 reports the potential of an angiotensin-converting enzyme 2 (ACE2)-antibody Fc complex in a new format that seems to offer more significant inhibition of SARS-CoV-2.
ACE2 has been one of the prime therapeutic and vaccine development targets because of its intrinsic role in viral attachment via the spike glycoprotein and entry into the host cell. Blocking this first step could prevent infection altogether, and is an easy intervention due to the location of the target on the surface of the cell, which avoids the need for cell entry.
Soluble ACE2 Receptors
Early research examined the use of soluble receptors for HIV-1, since viral binding to CD4 receptors via the envelope glycoprotein gp120competitively inhibits its attachment to the target CD4 T cell receptor, thereby prevents viral entry. In vitro studies confirmed this mechanism of viral inhibition.
When the protein was fused to the Fc portion of an immunoglobulin molecule, to form an immunoadhesin, gp120 dimers were formed, with significantly increased avidity. This was shown, in vivo, to result in a longer duration of protein survival in the body.
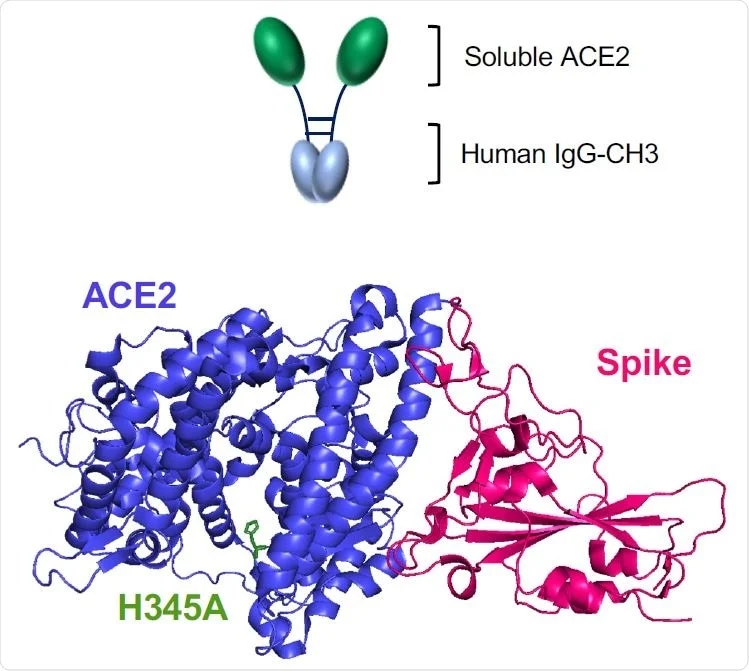
Later, another modification was made, with the addition of a viral coreceptor CCR5-derived peptide to the CD4-Ig immunoadhesin. This proved to be a potent viral inhibitor, protecting against infection in animal models.
The current paper deals with a similar soluble receptor approach to blocking SARS-CoV-2 entry, based on recombinant human soluble ACE2 protein (hrsACE2) or hrsACE2-IgG, which has the ACE2 bound to Ig-Fc. Both have been shown earlier to prevent infection with SARS-CoV and SARS-CoV-2 in a mouse model.
In phase 1 and phase 2 clinical trials, this protein showed partial blocking of viral activity but was quickly removed from circulation. The half-life improved in vivo with the addition of the Fc portion. However, this raises concerns as to the chances of severe disease due to antibody-dependent enhancement because of the presence of the Fc. The Fc receptor naturally occurs on immune cells and is the site of attachment of anti-spike antibody, leading to increased viral entry into these cells.
ACE2 Microbody Protein
To prevent this, the current researchers explored using a soluble human ACE2 “microbody” in which the ACE2 molecule is fused to the Ig heavy chain Fc through the ectodomain of the former. The ectodomain preserves the cysteine residues, allowing protein dimers to form, with a greater affinity of binding for the virus, even while reducing its molecular mass. The soluble ACE2 dimers and the ACE2 microbody dimers are held together by non-disulfide and disulfide bonds.
The researchers found that this microbody failed to bind to the Fc receptor on the immune cell surface and therefore, will not give rise to ADE. The antiviral activity of the microbody was ten times higher than that of soluble ACE2, though both were dimers. It also had a higher affinity for virion binding. In tissue culture, it persisted for a longer time than soluble ACE2.
It also blocked virus entry over a range of cell lines, for the original spike variant and the dominant D614G variant, as well as others in a panel of beta-coronavirus spike proteins.
Preventing ACE2 Catalytic Activity
The researchers found that introducing a specific H345A mutation in order to prevent the soluble ACE2 from exerting its catalytic enzymatic activity did not impact its affinity for the S protein. This is necessary to prevent its effects on blood pressure.
Pseudovirus Incorporates DS with Increased Infectivity
Pseudoviruses expressing a deletion mutant of the spike protein that removes the putative ER retention sequence that prevents the migration of the S protein to the cell surface had 20 or more times higher levels of S protein than those which expressed the full-length S protein. The number of virions was similar, however. The reason for the increased efficiency of DS packaging is thought to be the loss of inhibition of the ER cytoplasmic tail on S incorporation into the virion since both are almost equally expressed at the cell surface.
The researchers also found that pseudoviruses expressing the S protein specifically bound to the soluble ACE2 microbody proteins. This binding was inhibited by convalescent human serum with a high antibody titer. The mutant ACE2 microbody (both wildtype and soluble) bound to virions more efficiently than the wildtype microbody protein, even though the mutated amino acid is not at the surface, which interacts with the spike protein.
ACE2 Microbody Blocks SARS-CoV-2 Pseudovirus Infection
The investigators examined the ability of the soluble ACE2 and the ACE2 microbody to prevent SARS-CoV-2 DS pseudovirus replication, using a fluorescent dye marker. They found that while soluble ACE2 had moderate antiviral activity, the microbody was much more powerful, while the mutated microbody had highly potent inhibitory activity. The effective antiviral concentration EC50 was 1.24 μg/ml, 0.36 μg/ml and 0.15 μg/ml for the soluble ACE2, the ACE2 microbody and the mutated ACE2 microbody, respectively.
The activity of the soluble ACE2 is similar to that of convalescent serum and inhibits explicitly the SARS-CoV-2 spike protein.
They infected a cell line with live engineered SARS-CoV-2 containing a fluorescent dye marker gene in ORF7 so that its replication could be monitored. They found that at serial dilutions, the ACE2 microbody retained antiviral activity from 1-0.125 μg. With soluble ACE2, with an EC50 of 1 μg, the activity was lost at 0.5 μg. Both wild-type and mutated ACE2 microbody here showed similar EC50, being superior to soluble ACE2.
They then determined that the microbody proteins would be active when exposed to the virus at later time points as well, with the former being added simultaneously or up to 6 hours after infection. This resulted in 80% inhibition when both were added simultaneously, almost the same at 30 minutes from infection and 55% at 2 hours when about 10% of the infectious virus is bound to the cell. Activity waned after that.
Thus, the ACE2 microbody can block infection if present before virus binding to the target cell, or at the time of exposure, and even up to 2 hours following exposure. This time window indicates the period in which the virus has not yet significantly bound to the cell.
Moreover, even soon after the virus was bound, infection rates could be lowered by incubating with the ACE2 microbody for the next 30 minutes. The rate of virus binding to the ACE2 receptor was found to be time-dependent, with much more binding at 4 hours compared to 1 hour of incubation, perhaps because only a few spike-ACE2 interactions occur at first, followed by additional S proteins entering into the binding over time. As the latter occurs, the ACE2 microbodies are no longer able to inhibit viral entry.
ACE2 Microbody Blocks D614G Variant
A variant of SARS-CoV-2 containing a D614G point mutation in the S protein is known to become the dominant variant wherever it emerges rapidly. It is associated with increased infectivity. The DS pseudovirus containing this mutation is also blocked moderately by soluble ACE2, while both wildtype and mutant ACE2 microbody are more potent at inhibiting viral replication with this variant. A comparison of the two types of pseudoviruses concerning binding showed that soluble ACE2 binds the D614G spike-carrying variant more efficiently, while
ACE2 Microbody Binds to Other Betacoronavirus Spike Proteins
The ACE2 and H345A microbody proteins prevented the entry of all the pseudoviruses carrying different betacoronavirus spike proteins, indicating a broad spectrum of anti-betacoronavirus while soluble ACE2 had lower antiviral activity.
Implications
The ACE2 microbody is 10-fold more active than soluble ACE2 against SARS-CoV-2 and has a four-fold affinity to the viral particles. Its human origin means its immunogenicity is low. It also appears to have broad efficacy against emerging spike variants, including the D614G mutation.
The researchers introduced a specific mutation into the ACE2 microbody to eliminate the catalytic activity of the enzyme while preserving its antiviral activity. This mutation appeared to increase the activity of the microbody but not its inhibition potential in the live virus assay.
The researchers also suggest that the microbody may have more significant antiviral activity than observed here because most of the experiments were carried out on cells that express high levels of ACE2. When assayed on a cell line with very low ACE2 levels, the ACE2 microbody had more antiviral activity.
The investigators sum up, “The disulfide-bonded ACE2 microbody protein inhibited entry of lentiviral SARS-CoV-2 spike protein pseudotyped virus and live SARS-CoV-2 with a potency 10-fold higher than unmodified soluble ACE2 and was active after initial virus binding to the cell.”
The study also shows that the D614G S protein confers higher infectivity and a higher affinity for ACE2. However, the ACE2 microbody was able to neutralize the pseudovirus containing this spike variant, as well as other betacoronavirus spike proteins. This could indicate that this technology can be used to neutralize newly emerging coronaviruses that bind to the ACE2 receptor as well, enabling its use as “an off-the-shelf reagent that could be rapidly deployed.’
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
"entry" - Google News
September 21, 2020 at 12:33PM
https://ift.tt/2FPAXP0
Soluble ACE2 microbody blocks SARS-CoV-2 entry into lung cells - News-Medical.Net
"entry" - Google News
https://ift.tt/3f5ZAUJ
https://ift.tt/3d6LMHD
Bagikan Berita Ini














0 Response to "Soluble ACE2 microbody blocks SARS-CoV-2 entry into lung cells - News-Medical.Net"
Post a Comment