This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210128006046/en/
(Photo: Business Wire)
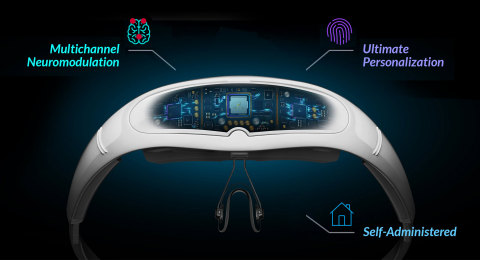
Relivion® is a non-invasive brain neuromodulation* device that is placed around the head and delivers unparalleled stimulation to the occipital and trigeminal nerves. This creates a cumulative effect by releasing neurotransmitters in the brainstem and modulating brain networks associated with control of pain and mood. The device is designed to allow patients to use Relivion® at home, and share treatment data with their physician, in addition to uploading the data to the cloud database through a dedicated app. It is also designed to self-learn and analyze the treatments using AI technology in order to optimize the treatments for patients according to their symptoms.
*Neuromodulation: A therapeutic technology to modulate neurological activities through the administration of electric/magnetic stimuli or drugs.
With the view to launch Relivion in Japan, submissions to the Pharmaceuticals and Medical Devices Agency (PMDA) are expected for migraine by 2022 and depression by 2023. If Relivion® is approved by the agency, it will be the only brain neuromodulation device available for treatment at home in Japan.
*In Europe, Neurolief obtained the CE Mark certification as a treatment device for migraine and is in preparation for the market launch. In the US, the application was filed to FDA in November 2020 as a 510(K) submission for migraine and is scheduled to be filed for depression by 2022.
By working with Sawai to introduce the Relivion® neuromodulation device in Japan for use at home with doctor's supervision, we are widening treatment options for patients who are suffering from either migraine or depression.
About Neurolief
Dedicated to bringing relief to patients suffering from chronic neurological and neuropsychiatric disorders, Neurolief is creating a digital therapeutics platform of wearable clinically-proven neuromodulation solutions. This technology, which is made to be worn like a headset, is intended to offer highly effective, safe treatment options that work with current pharmaceutical therapies or may provide an alternative to these therapies. It is designed to concurrently neuromodulate major neural pathways in the head, and thereby affect brain regions that are involved in control and modulation of mood and pain. Neurolief’s technology is currently studied for patients with migraine and major depression, and future indications may include insomnia, ADHD and additional chronic pain and neuropsychiatric disorders. The company is based in Israel, with US operations in Tampa, FL and is made up of highly experienced professionals with a proven track record in neurosciences, neuromodulation technology and the neurotech devices industry. For more information, visit https://www.neurolief.com/
*The Trademark application for Relivion in Japan has been filed by Neurolief.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210128006046/en/
"enter" - Google News
January 29, 2021 at 03:35AM
https://ift.tt/3oolAgQ
Neurolief and Sawai Enter into Exclusive Development and Marketing Agreement for Relivion® - BioSpace
"enter" - Google News
https://ift.tt/2TwxTMf
https://ift.tt/3d6LMHD
Bagikan Berita Ini














0 Response to "Neurolief and Sawai Enter into Exclusive Development and Marketing Agreement for Relivion® - BioSpace"
Post a Comment